What are cannabinoids?
Cannabinoids are chemical compounds referred to as terpenes, produced in plant glands called trichomes. They are only found in female flowers. So far, over 100 types of them have been identified[1].
They occur naturally in plants in the form of acids, which also have biological properties, but the active forms, are obtained during the decarboxylation process.
The main and dominant phytocannabinoids are - tetrahydrocannabinol acid (THCa), cannabidiol acid (CBDa), cannabinol acid (CBNa), cannabidivarin acid (CBDVa) and cannabichromene acid (CBCa).
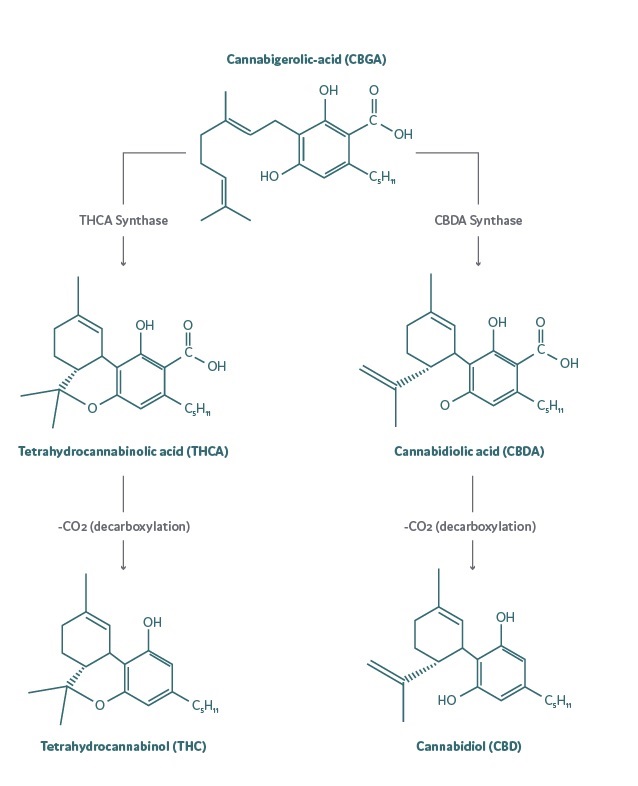
Fig. The process of synthesis and decarboxylation of THCa and CBDa to THC and CBD, respectively.
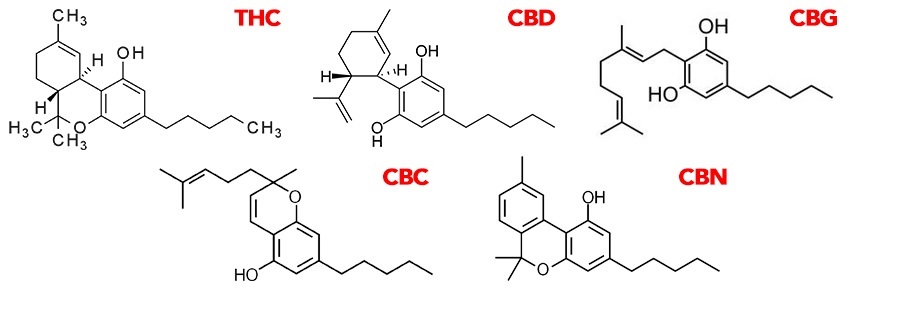
Fig. Chemical structure of selected decarboxylated cannabinoids.
According to their origin, cannabinoids can be divided into the aforementioned phytocannabinoids, mammalian-produced endocannabinoids and synthetic cannabinoids developed for therapeutic purposes by pharmaceutical companies.
Regardless of their origin, they act through the cannabinoid receptor system, which includes CB1, CB2, TRPV1 and GPR55 receptors [2].
CB1 and CB2 receptors belong to the family of G protein-related receptors their mechanism of action is also related to the influence on calcium and potassium channels [3,4]. CB1, located in cell membranes of presynaptic cells of both central and peripheral nervous systems their activation results in inhibition of neurotransmitters release in, e.g. noradrenergic, dopaminergic, serotonergic pathways, as well as those connected with acetylcholine, gamma-aminobutyric acid and hormones[5]. They have also been found in testicular tissues, uterus, vascular endothelium, spleen, intestines, adipocytes and on the surface of T lymphocytes[6,7]. CB2 receptors, on the other hand, are located mainly on the surface of immune cells, especially B lymphocytes, macrophages and monocytes, NK cells and T lymphocytes. Moreover, CB2 expression has been shown in dendritic cells, microglia, thymus and spleen[8]. Stimulation of these receptors induces an immunomodulatory effect by influencing the release of cytokines (increase of anti-inflammatory, inhibition of pro-inflammatory)[9].
The vanilloid receptors TRPV1 (Transient Receptor Potential cation channel subfamily V member 1) are mainly localized in the cell membrane of sensory afferent neurons such as dorsal root ganglia, trigeminal ganglia and nerve fibres [10]. Activation of TRPV1 leads to regulation of blood flow, body temperature and is responsible for insulin and cytokine release [11].
On the other hand, the GPR55 orphan receptor, also associated with G protein, is located in the brain, liver, spleen, cardiovascular and intestinal cells [12]. Significantly, it exhibits the highest expression in malignant tumour cells[13]. Its activation results in modification of the number and function of osteoclasts and increased proliferation of tumour cells. It is presumed that GPR55 is also responsible for the regulation of vascular tone[14].
The first and best-known endocannabinoids include: N-ara-chidonylethanolamide called anandamide (AEA) and 2- arachidonyl-glycerol (2-AG).
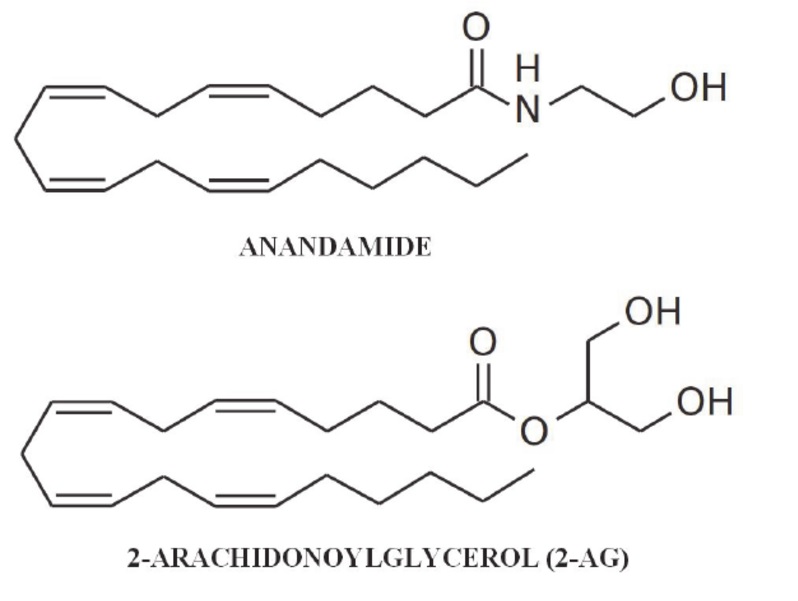
Fig. Chemical structure of AEA and 2-AG
Anandamide is produced out of cell membrane phospholipids, with the highest concentrations found within brain tissue. Due to its high concentration in brain tissue, it is known as a cannabinoid receptor agonist, mainly CB1 but also, to a lesser extent, CB2 and TRPV1. Activation of CB1, by inhibiting adenylyl cyclase activity, has an antiproliferative effect, increases the formation of active oxygen species and activation of caspase-3 resulting in increased apoptosis of tumour cells[15] It also influences the increase in ceramides and activation of the Raf1/ERK kinase cascade [16] and JNK/p38 kinases[17] Furthermore, activation of CB2 causes an increase in ceramides resulting in through the expression of p28 protein and transcription factor 4, results in an apoptotic effect [18].
By stimulating the TRPV1 receptor, AEA contributes to enhanced oxidative stress and calpain activation, which also leads to apoptosis[19]
2-arachidonyl-glycerol is also predominantly concentrated in brain tissues, with concentrations nearly 800 times higher than AEA[20], and binds to CB1 and CB2 receptors more readily than anandamide[21]. Activation of CB2 results in antioxidant and anti-inflammatory effects by reducing the production of the cytokine Il-17[22], while attachment to CB1 results in inhibition of cancer cell growth and proliferation[23]. Moreover, 2-AG is involved in the activation of signal transduction pathways in the endocrine and immune systems and participates in the production of nitric oxide and PGE2 through induction of NOS and COX-2 [24].
The multidirectional action of phyto- and endocannabinoids, as well as those of synthetic origin (Nabilon, Dronabilol preparations), is determined by the presence of numerous receptors, their wide distribution throughout the body and the complex mechanisms of action.
Bibliografia:
[1]Fischedick, J. T., Hazekamp, A., Erkelens, T., Choi, Y. H., and Verpoorte, R. (2010). Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 71, 2058–2073. doi: 10.1016/j.phytochem.2010.10.001
[2]Alexander S.P.: Therapeutic potential of cannabis related drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry, 2016; 64: 157 166
[3]Glass, M., & Northup, J. K. (1999). Agonist Selective Regulation of G Proteins by Cannabinoid CB1and CB2Receptors. Molecular Pharmacology, 56(6), 1362–1369. doi:10.1124/mol.56.6.1362
[4]Caulfield MP and Brown DA (1992) Cannabinoid receptor agonists inhibit Ca21
current in NG108–15 neuroblastoma cells via a pertussis toxin sensitive mechanism. Br J Pharmacol 106:231–232.
[5] Pacher P., Bátkai S., Kunos G.: The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev., 2006; 8: 389-462
[6]8. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325.
[7]Borner, C., Bedini, A., Hollt, V., Kraus, J., 2008. Analysis of promoter regions regulating basal and interleukin-4-inducible expression of the human CB1 receptor gene in T lymphocytes. Mol. Pharmacol. 73, 1013–1019.
[8]Galiegue, S., Mary, S., Marchand, J., Dussossoy, D., Carriere, D., Carayon, P., Bouaboula, M., Shire, D., Le Fur, G., Casellas, P., 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61
[9] Pacher P., Bátkai S., Kunos G.: The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev., 2006; 8: 389-462
[10]Kim Y.H., Back S.K., Davies A.J., Jeong H., Jo H.J., Chung G., Na H.S., Bae Y.C., Kim S.J., Kim J.S., Jung S.J., Oh S.B..: TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron, 2012; 74: 640-647
[11]Premkumar L.S., Abooj M.: TRP channels and analgesia. Life Sci., 2013; 92: 415-424
[12]Li K., Fichna J., Schicho R., Saur D., Bashashati M., Mackie K., Li Y., Zimmer A., Göke B., Sharkey K.A., Storr M.: A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology, 2013; 71: 255-263
[13]Andradas C., Caffarel M.M., Pérez-Gómez E., Salazar M., Lorente M., Velasco G., Guzmán M., Sánchez C.: The orphan G protein-coupled Piśmiennictwo receptor GPR55 promotes
[14]Ryberg, E. , Larsson, N. , Sjögren, S. , Hjorth, S. , Hermansson, N. , Leonova, J. , Elebring, T. , Nilsson, K. , Drmota, T. and Greasley, P. J. (2007), The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152: 1092-1101. doi:10.1038/sj.bjp.0707460
[15]Cianchi F., Papucci L., Schiavone N., Lulli M., Magnelli L., Vinci M.C., Messerini L., Manera C., Ronconi E., Romagnani P., Donnini M., Perigli G., Trallori G., Tanganelli E., Capaccioli S., Masini E.: Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res., 2008; 14: 7691-7700
[16]Andre C. M., Larondelle Y., Evers D. (2010). Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr. Nutr. Food Sci. 6 2–12. 10.2174/157340110790909563
[17]Rueda D., Galve-Roperh I., Haro A., Guzman M.: The CB1 cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol. Pharmacol., 2000; 58: 814-820
[18]Carracedo A., Gironella M., Lorente M., Garcia S., Guzmán M.,Velasco G., Iovanna J.L.: Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res., 2006; 66: 6748-6755
[19]Jacobsson S.O., Wallin T., Fowler C.J.: Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J. Pharmacol. Exp. Ther., 2001; 299: 951-959
[20]Bradshaw H.B., Rimmerman N., Krey J.F., Walker J.M.: Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am. J. Physiol. Regul. Integr. Comp.
Physiol., 2006; 291: R349-R358
[21]Steffens M., Feuerstein T.J., Van Velthoven V., Schnierle P.,Knörle R.: Quantitative measurement of depolarization-induced anandamide release in human and rat neocortex. Naunyn-Schmiedebergs
Arch. Pharmacol., 2003; 368: 432-436
[22]Sugiura T., Kishimoto S., Oka S., Gokoh M.: Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid. Res., 2006; 45: 405-446
[23]Liao Y.S., Wu J., Wang P., Zhang H.: Anandamide inhibits the growth of colorectal cancer cells through CB1 and lipid rafts. Zhonghua Zhong Liu Za Zhi, 2011; 33: 256-259
[24]Vannacci A., Giannini L., Passani M.B., Di Felice A., Pierpaoli S., Zagli G., Fantappie O., Mazzanti R., Masini E., Mannaioni P.F.: The endocannabinoid 2-arachidonylglycerol decreases the immunological activation of guinea pig mast cells: involvement of nitric oxide and eicosanoids. J. Pharmacol. Exp. Ther., 2004; 311: 256-264











